Introduction of GCP Center of Xiyuan Hospital
National Good Clinical Practice Center (hereinafter referred to as GCP Center) is one of the 14 national drug clinical pharmacology bases under the Ministry of Health, which was firstly approved by the Ministry of Health in 1983, and it is a GCP platform construction unit from the National 9th Five-Year Plan to 13th Five-Year Plan. It is the national engineering center for clinical efficacy and safety evaluation of TCM, the key laboratory of the National Medical Products Administration, the Beijing Demonstration Research Ward, the Research Institute of Clinical Pharmacology of the CACMS, the Key Discipline of Clinical Pharmacology of TCM of the State Administration of Traditional Chinese Medicine and the Beijing Clinical Research Quality Promotion Center. Adhering to the spirit of "scientific methodology, advanced technology, standardized operation, perfect management and high quality service", the GCP Center has set up a "one-stop" platform for the whole chain of clinical trial services, and has signed contracts with more than 60 domestic and foreign pharmaceutical companies to carry out more than 300 clinical trials of new traditional Chinese medicine, of which 20 have been conducted. We have contracted with more than 60 pharmaceutical companies at home and abroad to conduct more than 300 clinical trials for new Chinese medicines, of which more than 20 have obtained new drug certificates. The company has realized a multi-link and multi-dimensional electronic and intelligent management mode of clinical trials with the clinical trial intelligent management platform as the management core, CTMS and TrialOne System as the clinical trial data core, and clinical trial remote monitoring management platform as the quality control core, thus guaranteeing high-quality and high-efficiency clinical trials.


Institute of Clinical Pharmacology, Xiyuan Hospital of CACMS
Organization Chart
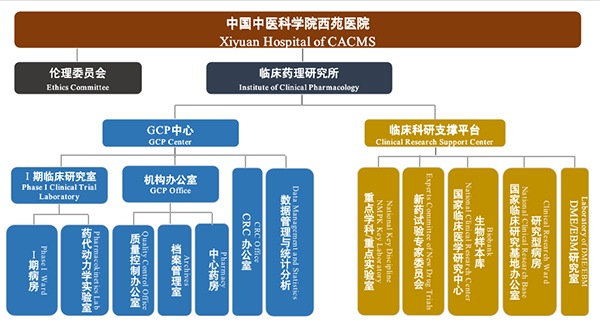
Contact Information
GCP Office 010-62835649 Mr. Shi; 010-62835650 Mr. Qiu
Ethics Committee 010-62835646 Ms. Jia
Phase I Clinical Trial Laboratory 010-62862428 Ms. Li
For more information about the office flow and professional introduction, please refer to the official website of the organization: https://xyyygcp.wetrial.com/
Source: Institute of Clinical Pharmacology, Xiyuan Hospital of CACMS
English Editor/Translator: Zhang Fangteng
Chinese Editor/Proofreader: Jia GuanChun